Sinclair Dermatology has conducted more than 100 global clinical trials that investigate new treatments for dermatological conditions. A number of these trials have successfully progressed to TGA approval for treatment and made easily accessible to the public. Our team is proud to be involved in advancing dermatology treatment and continue to strive for better future health outcomes.
Our dedicated clinical trials team are experts in conducting Phase I through to Phase IV dermatological clinical trials. These include studies in promising medications and medical devices. Our experience and high level of commitment to each trial allows for the collection of valuable research data and further the development of potential treatments for a vast range of skin, hair and nail conditions.
Our state-of-the-art facilities include private clinical consultation rooms, specialised photography and an in-house pharmacy. Our clinical trials team are focused on ensuring the best patient experience.
Current Clinical Trials
To register your interest in one of our trials please click here to complete a brief eligibility questionnaire.
One of our recruitment specialists will be in touch shortly. All trials are open to Victorian residents. Treatment and study care will be provided free of charge to eligible participants during the course of the trial.
Alopecia Areata
CONDITION
AGE
SEVERITY
STATUS
REGISTRATION
Alopecia Areata – Adolescents and Adults
Individuals who have a diagnosis of severe alopecia areata (AA) may qualify for this clinical research trial and receive investigational oral medication or placebo.
- Age: ≥ 12 years and ≤ 65 years old
- 50% or more scalp hair loss
- Current AA episode less than 8 years
- No spontaneous scalp hair regrowth over the past 6 months
Patients will be asked to visit the clinic about 16 times over approximately 160 weeks. There is no cost to participate, and participants will be reimbursed for their time and travel.
For further information on the study, please follow the link:
Trial ID: NCT06012240

Alopecia Areata – Adults
Adults who have a diagnosis of severe alopecia areata (AA) may qualify for this clinical research trial and receive investigational subcutaneous injection or placebo.
- Age: ≥18 years old
- AA diagnosis ≥ 1 year
- 50% or more scalp hair loss
- Current AA episode: ≥ 6 months and ≤ 8 years
- Willing to maintain hair style and hair care such as products, wigs, and extensions. Unable to shave scalp hair 2 weeks before visits.
Patients will be asked to visit the clinic about 12 times over approximately 54 weeks. There is no cost to participate, and participants will be reimbursed for their time and travel.
For further information on the study, please follow the link:
Trial ID: NCT06444451
Atopic Dermatitis
CONDITION
AGE
SEVERITY
STATUS
REGISTRATION

Atopic Dermatitis – Adolescents and Adults
Adolescents and adults who have a diagnosis of moderate to severe atopic dermatitis (AD) may qualify for this clinical
research trial and receive investigational medication or placebo.
• Age ≥ 12
• AD diagnosis ≥ 1 year
• moderate to severe atopic dermatitis (EASI score ≥ 16)
• AD involvement of 10% or more of body.
• Documented inadequate response to systemic or biologic therapies
Patients will be asked to visit the clinic about 13 times over a 52-week period. There is no cost to participate, and
participants will be reimbursed for their travel and time.
For further information on the study, please follow the link:
Trial ID: NCT06241118
Psoriasis
CONDITION
AGE
SEVERITY
STATUS
REGISTRATION
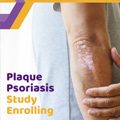
Plaque Psoriasis – Adults – Phase 4
Adults who have a diagnosis of moderate to severe plaque psoriasis (plaque PsO) may qualify for this clinical research trial and receive investigational oral medication or placebo.
- Age: ≥ 18
- BSA ≥ 10%, PASI ≥ 12, sPGA ≥ 3
- Diagnosis of Plaque PsO within the past 6 months
Patients will be asked to visit the clinic about 11 times over approximately 32 weeks. There is no cost to participate, and participants will be reimbursed for their time and travel.
For further information on the study, please follow the link:
Trial ID: NCT06846541
If you are interested and would like to participate in this trial, please register your details and answer a few short questions for our recruitment team to back to you: eligibility questionnaire
Vitiligo
CONDITION
AGE
SEVERITY
STATUS
REGISTRATION

Vitiligo – Adults
Adults who have a diagnosis of non-segmental vitiligo may qualify for this clinical research trial and receive investigational medication or placebo.
- Age: ≥ 18 years old
- Vitiligo for ≥ 3 months
- BSA ≥ 4% and facial BSA ≥ 0.5%
- All Fitzpatrick skin types
- No previous exposure to JAK inhibitors
Patients will be asked to visit the clinic about 17 times over approximately 104 weeks. There is no cost to participate, and participants will be reimbursed for their time and travel.
For further information on the study, please follow the link:
Trial ID: NCT06072183
Hidradenitis Suppurativa
CONDITION
AGE
SEVERITY
STATUS
REGISTRATION

Hidradenitis Suppurativa (HS) – Adolescents and Adults
AGE12+
SEVERITYModerate to Severe
STATUSOPEN
Hidradenitis Suppurativa – Adolescents and Adults
Individuals who have a diagnosis of moderate to severe hidradenitis suppurativa (HS) may qualify for this clinical research trial and receive investigational oral medication or placebo.
- Age: ≥ 12 years old
- Diagnosis of HS ≥ 6 months
- HS lesions in ≥ 2 distinct anatomical areas and total lesion count ≥ 5
- Inadequate response or intolerance to anti-TNF (ie. Humira)
Patients will be asked to visit the clinic about 24 times over approximately 104 weeks. There is no cost to participate, and participants will be reimbursed for their time and travel.
For further information on the study, please follow the link:
Trial ID: NCT05889182

Hidradenitis Suppurativa – Adolescents and Adults
Adults who have a diagnosis of moderate to severe hidradenitis suppurativa (HS) may qualify for this clinical research trial and receive investigational injectable medication or placebo.
- Age: ≥ 18 years old
- Diagnosis of HS ≥ 12 months
- HS lesions in ≥ 2 distinct anatomical areas and total lesion count ≥ 5
- Inadequate response or intolerance to 28-day course of oral antibiotics
Patients will be asked to visit the clinic about 31 times over approximately 64 weeks. There is no cost to participate, and participants will be reimbursed for their time and travel.
For further information on the study, please follow the link:
Trial ID: NCT06046729

Hidradenitis Suppurativa (HS) – Adolescents and Adults
AGE16+
SEVERITYModerate to Severe
STATUSOPEN
Hidradenitis Suppurativa – Adolescents and Adults
Adults and adolescents who have a diagnosis of moderate to severe hidradenitis suppurativa (HS) may qualify for this clinical research trial and receive investigational injectable medication or placebo.
- Age: ≥ 16 years old
- Diagnosis of HS ≥ 6 months
- HS lesions in ≥ 2 distinct anatomical areas and total lesion count ≥ 5
- Inadequate response or intolerance to a 12-week trial of oral antibiotics
Patients will be asked to visit the clinic about 19 times over approximately 52 weeks. There is no cost to participate, and participants will be reimbursed for their time and travel.
For further information on the study, please follow the link:
Trial ID: NCT06468228
Urticaria
CONDITION
AGE
SEVERITY
STATUS
REGISTRATION
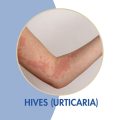
Chronic Spontaneous Urticaria – Adults
Adults who have Chronic Spontaneous Urticaria (hives) may qualify for this clinical research trial and receive investigational injectable medication or placebo.
- Age ≥ 18
- CSU diagnosis ≥ 6 months
- Persistent hives and/or angioedema for ≥ 6 weeks consecutively at any time after, despite treatment with a second-generation H1 antihistamine (H1AH)
- Documented use of a stable dose of a H1AH ≥ 4 weeks
Participants will be asked to visit the clinic about 19 times over a 72-week period. There is no cost to participate, and participants will be reimbursed for their travel and time.
For further information on the study, please follow the link
Trial ID: NCT06445023
What Is a Clinical Trial?
Clinical trials (also known as clinical studies) are conducted to learn about the safety and effectiveness possible biomedical interventions (new medications, medical devices, and medical procedures) can have on people in the real world.
Clinical trials are essential to the development of new interventions. For example, without clinical trials, we cannot properly determine whether new medicines developed in the laboratory or by using animal models are effective or safe, or whether a diagnostic test works properly in a clinical setting. This is because computer simulation and animal testing can only tell us so much about how a new treatment might work and are no substitute for testing in a living human body.
Clinical trials also permit testing and monitoring of the effect of an intervention on a large number of people to ensure that any improvement as a result of the intervention occurs for many people and is not just a random effect for anyone.
Most modern medical interventions are a direct result of clinical research. New interventions for most diseases and conditions have been developed through clinical research. Clinical trials often lead to new interventions becoming available that help people to live longer and to have less pain or disability .
Source: https://www.australianclinicaltrials.gov.au/what-clinical-trial

Phases of Clinical Trials
Clinical trials of biomedical interventions (new medications) typically proceed through four different stages, which are called "phases". In the early phases, the new intervention is tested in a small number of participants to assess safety and effectiveness. If the intervention is promising, it may move to later phases of testing where the number of participants are increased to collect more information on effectiveness and possible side effects. All new drugs must be tested in phase I, II and III studies before being approved for use by the public. Phase IV is done after the drug has been approved by the Therapeutic Goods Administration (TGA).
Preclinical
Test how a body responds to an experimental drug. In these studies, small doses of the new drug are tested in the research laboratory or in small animals.
Phase 1
>20-80 people
Evaluate how safe the medicine is and how it interacts with the human body. Typically, these trials are very short, and very tightly controlled with a high degree of monitoring of participants.
Phase 2
>100 people
Determine the efficacy (that is, whether it works as intended) and to further evaluate its safety in a larger group of people. Typically, these trials are placebo-controlled, fairly short and participants are closely monitored.
Phase 3
>1000 people
Further evaluate safety and efficacy of an intervention in large groups of trial participants comparing the new drug to placebo or other standard interventions for the condition. Participants are monitored closely.
Phase 4
TGA approved
& Marketed
Monitor the effectiveness of the approved intervention in the general population and to collect information about any adverse effects associated with widespread use over longer periods of time.
Sinclair Dermatology is committed to excellence in patient care, clinical research and to the
development of new treatments that improve our patients' lives. We conduct trials at different phases.
For more information on clinical trial phases, please visit: https://www.australianclinicaltrials.gov.au/what-clinical-trial/phases-clinical-trials
Types of Clinical Trials
Although there are different types of clinical trials, all must conform to strict rules set by country-specific regulatory. In Australia, the bodies responsible for the regulation for assessing and monitoring clinical trials are the Human Research Ethics Committees (HREC), the pharmaceutical companies running the clinical trials and the Therapeutic Goods Administration (TGA).
Sinclair DIRECT works with local and international pharmaceutical companies to conduct trials in a broad range of skin conditions. All research is conducted in accordance with International Conference on Harmonisation-Good Clinical Practice (ICH-GCP) guidelines. Ensuring the safety and comfort of all our trial participants is our upmost priority.
Sponsor-led Trials
Sponsor-led trials are clinical trials that are initiated by drug or device companies who have developed a new treatment. The companies or also known as “Sponsors” engage a contract research organisation (CRO) to act on their behalf to ensure the trial runs smoothly and in accordance with ICH-GCP guidelines.
Investigator-led Trials
Investigator-led trials are clinical trials initiated by individual or groups of health care practitioners such as doctors or medical research entities. These trials may be funded by a research organisation, grants from research funding bodies, or by the doctors themselves.
Participants Journey

Expression of interest and pre-screening/phone screening
- Complete online questionnaire to express your interest in a study or to discuss eligibility with one of our Study Coordinators (SC).
- When a suitable trial is identified, we can book you in for an assessment with one of our Study Doctors. Your SC will inform you of any documents you may need to bring.

Screening appointment on site at Sinclair Dermatology
- Chat with our Study Doctor about the study and ask any questions you may have.
- If you are still interested, we will conduct a formal consent process.
- A thorough assessment will be conducted, we will chat with you about your current and prior medication your health history and any other requirements for the study.

Commencement of
trial participation
- At this visit you will undergo final review of eligibility. If all eligibility requirements are met, you will receive your first study medication.
- This may be a long visit as we’d like to stay around after your first dose to make sure you are OK.

Reimbursement
- There is no cost to you to participate in a clinical trial
- For most studies, a reimbursement of your time and travel expenses will be provided.
Why Should I Participate In a Clinical Trial?
Clinical trials offer the hope of developing better interventions or tests for a particular disease or condition. Even if a trial does not provide a benefit for an individual, it may provide benefits for others with the disease in the future. Clinical trials often lead to new interventions becoming available that help people to live longer and to have less pain or disability.
Some of the possible advantages of participating in a clinical trial may include:
- to be at the forefront of medical research
- potentially finding relief for your condition long before successful treatments become readily available
- receive all medication and regular medical checks free of charge during the study
- contribute to the advancement of scientific knowledge that could help many others with the same disease or condition now and in the future
- a way to give back to the community
If you are interested in participating in one of our clinical trials please answer a few short questions via the below link and one of our recruitment co-ordinators will be in contact with you shortly: eligibility questionnaire.
Common Questions
Each clinical drug trial and most device trials are led by a doctor who is referred to as the “Principal Investigator”. The clinical trial team includes doctors, nurses, pharmacists, study coordinators and trial assistants. The clinical trial team is responsible for conduct of the whole trial.
If you choose to participate in a clinical trial, you will receive all trial-related care at no cost including any visits to your study doctor’s office. You do not need insurance to participate.
The duration of your participation varies depending on the trial. Before you decide to participate, your dedicated study team will provide you with a detailed summary of what will happen at each visit, and answer any questions you may have related to the trial. This is so that you can make an informed decision about joining the trial.
At every visit, you will be asked about how you are feeling, and your study doctor will examine you. The study team will also review with you how to apply or use the study treatment. At these visits, you will receive a routine physical exam and any other trial procedures will be conducted.
If a trial you are participating in requires you to take any time off work, you will be provided with a certificate of attendance. You may also be eligible to receive a travel reimbursement each time you attend the clinic for a trial-related appointment.
As with all clinical trials, your participation is completely voluntary. You may leave the trial at any time without any effect on your future medical care.
Once available the results may be made available and may be published in medical journals and other relevant publications. Participants can obtain a written plain language explanation of the results from the Principal Investigator.
If a trial you are participating in requires you to take any time off work, you will be provided with a certificate of attendance. You may also be eligible to receive a travel reimbursement each time you attend the clinic for a trial-related appointment.
All clinical trials are conducted in accordance with the Victorian Health Record’s Act Privacy Principles. Your information will be kept confidential and you will not be able to be identified in any publication of the results. Your consent to share your information with any other organisation will be obtained before information is ever released.
Our clinical trials team is always available to answer any information that you do not understand and encourage you to reach out to them for an explanation.
About Sinclair DIRECT
In 2010, Sinclair Dermatology established Australia’s largest dedicated Dermatology Investigational Research Education, and Clinical Trials Centre (DIRECT) to reinforce its commitment to excellence in patient care, clinical research and to the development of new treatments to improve patients’ lives.
DIRECT is Australia’s only dedicated dermatology clinical research centre and the leader in dermatology education for dermatologists, dermatology trainees, general practitioners, medical students, other medical professionals and involved in the 2021 World Congress of Hair Research and the International Society of Dermatology World Congress.
Since its formation, DIRECT has conducted more than 100 sponsored Phase I, Phase II, Phase III and Phase IV clinical trials in the following dermatological conditions:
- Acne
- Alopecia areata
- Atopic dermatitis
- Basal cell skin cancer
- Chemotherapy-induced hair loss
- Melanoma detection/mole and skin scanner
- Male pattern hair loss
- Female pattern hair loss
- Frontal fibrosing alopecia/lichen planopilaris
- Hidradenitis suppurativa
- Pemphigus vulgaris
- Psoriasis
- Psoriatic arthritis
- Rosacea
- Urticaria
- Vitiligo
All research conducted at Sinclair DIRECT are in accordance with the ICH Good Clinical Practice Guidelines and the protocol for the conduct of clinical trials has been examined and approved by Human Research Ethics Committee. DIRECT has been inspected by the European Medicines Agency (EMA) with no critical findings as well as having participated in number of sponsor- initiated audits, with no critical findings.
DIRECT is the only clinical trials centre in the world to have the Canfield VECTRA WB360 whole-body skin imaging system, which was designed specifically for dermatology. The VECTRA WB360 is invaluable for helping calculate affected skin surface areas and monitors changes to a participant’s condition over time.
DIRECT@ Sinclair Dermatology works with local and international pharmaceutical companies to conduct sponsored clinical trials in a broad range of skin diseases. DIRECT also conducts investigator-initiated clinical studies and fundamental research into skin biology and disease mechanisms. We bring together the best team of more than 20 laboratory and clinical researchers, who are all dedicated to improving the lives of people affected by skin disease. We strive to provide better treatments, better care and progressively moving towards finding a cure for skin diseases and skin cancer.
About Our Investigational Research
Our laboratory research involves gene discovery, cell culture and stem cell biology and hair follicle cloning. We have published more than 500 papers in high-impact journals including the New England Journal of Medicine, The Lancet, Nature Genetics, British Medical Journal, EMBO, Journal of Investigative Dermatology, Experimental Dermatology, British Journal of Dermatology, Journal of the American Academy of Dermatology, international Journal of Dermatology, Journal of the European Academy of Dermatology and Australasian Journal of Dermatology.
Team

Prof. Rod Sinclair
Research Director, Principal Investigator & Principal Dermatologist

Dr Samantha Eisman
Principal Investigator & Consultant Dermatologist

Dr Adam Daunton
Principal Investigator & Consultant Dermatologist

Dr Bevin Bhoyrul
Principal Investigator & Consultant Dermatologist

Dr Nicole Yoong
Sub Investigator

Dr Valerie Yii
Sub Investigator

Dr Meghana Paranjape
Sub Investigator & Dermatology Fellow

Dr Flavia Rodrigues-Dias
Sub Investigator & Dermatology Fellow

Laita Bokhari
Head of Clinical Trials & Research

Dr Carol Robinson
Contracts Manager

Irina Danilovich
Operations Manager

Sophie Morton
Finance Manager
Team
The trials unit has clinical trials fellows and an experienced team of clinical trials coordinators, nurses and assistants who assist in the operation of the department.
DIRECT is Australia’s only dedicated dermatology clinical research centre and the only clinical trials centre in the world to have the Canfield VECTRA WB360 whole-body skin imaging system, which was designed specifically for dermatology. The images produced provide sponsors with the most accurate information possible on clinical response.
Our management team oversees the centre that comprises doctors, nurses, pharmacists, clinical trial coordinators and assistants.
- Research Director & Principal Investigator: Prof. Rod Sinclair (MBBS, MD, FACD)
- Principal Investigator: Dr Samantha Eisman (MBChB, FACD)
- Clinical Trials Unit Manager: Dr Carol Robinson (PhD)
- Research Scientist & Projects Manager: Ms Laita Bokhari (MPhilMed)
- Clinical Trials Operations Manager: Ms Megan Defazio
Facilities
We provide high-quality facilities and equipment for on-site monitoring
- Three dedicated consulting rooms
- Conference/meeting room
- Secure authorised access to clinical trials administration area
- Dedicated locked cold-chain monitored refrigerators (ambient temperature, 2-8°C) and freezers (-20°C and -80°C)
- Electrocardiogram (ECG)
- Centrifuge
- Incubators
- Dedicated sample processing area
- Use of electronic and paper records
- High-speed internet
Capabilities
- All research conducted at Sinclair DIRECT are in accordance with the ICH Good Clinical Practice Guidelines
- The protocol for the conduct of clinical trials has been examined and approved by Human Research Ethics Committee.
- Sinclair DIRECT has been inspected by the European Medicines Agency (EMA) with no critical findings
- Sinclair DIRECT has participated in a number of sponsor-initiated audits, with no critical findings
- Sinclair DIRECT regularly surpasses recruitment targets
Companies We Have Worked With











Accreditations & Memberships





















Clinical Trials Unit Opening Hours
Mon: 8:30am – 4:30pm
Tue: 8:30am – 4:30pm
Wed: 8:30am – 4:30pm
Thu: 8:30am – 4:30pm
Fri: 8:30am – 4:30pm
Sat: Closed
Sun: Closed


