For some, acetone nail polish remover is the big bad wolf of the nail industry.
You know. The bottles of stringent-smelling liquid you soak cotton wool balls in to remove that red toenail polish you’ve been painting over for 6,876,980 years.
Google ‘is acetone bad’ and you’ll find lots of articles talking about the damage acetone polish removers can do to your nails, but when you ask the experts, they’ll tell you there’s no hard science proving acetone nail polish remover is dangerous.
But what actually is acetone, what does it do to your nails and is it safe to use? And if acetone is ‘bad’, are there other ways to remove nail polish, acrylic nails, SNS nails and shellac without it?
To find out, we asked a dermatologist specialising in nail health, and two nail technicians to explain what the fuss is around acetone and if it’s fine to keep using it.
What is acetone and is it bad for nails? A summary…
- Acetone is a solvent that can be found in nail polish removers.
- Acetone polish remover works by breaking down nail polish and removing it from the nail plate surface.
- Acetone isn’t toxic, but it is dangerous when ingested.
- Exposure to acetone can dehydrate the nail plate, cuticles and the surrounding skin – nails can become dry and brittle, and cuticles can become dry, flaky, red and irritated.
- Non-acetone nail polish removers also contain chemicals and natural nail polish removers require scrubbing to remove polish, which can damage the nails.
- Non-acetone solvent solutions can be used to remove acrylic, gel, shellac and SNS manicures.
- There is no conclusive research to suggest acetone is harmful or toxic – it is the most effective nail polish remover currently on the market.
- Infections from poor salon hygiene, allergic contact dermatitis and UV lamps are all more concerning nail issues than acetone nail polish remover.
What is acetone/what is acetone polish remover?
“Acetone, also known as propanone, is a colourless, flammable liquid. It’s a solvent (dissolves substances) and is used in the manufacturing of plastics, household products, cosmetic and personal care products,” Sinclair Dermatology’s Dr Samantha Eisman told Mamamia.
“Acetone is also produced in the human body and is normally present in the blood and urine, as a by-product of metabolism.”
The Nail Lab Nail Technician Thea Phan added, “In the beauty industry, acetone is great for removing nail polish, gel colour and acrylic nails, and prepping the nail for a new coat of nail polish. Salons normally use a 70- 90 per cent acetone solution during manicures.”
How does acetone in polish remover work?
Traditional nail polish removers are made up of an acetone solvent and a fatty material like lanolin or caster oil. Acetone removes polish by quickly breaking apart the nail varnish and stripping the polish from the nail plate surface.

Is acetone toxic?
Short answer, no.
“Acetone is not registered as a carcinogen (cancer-causing) and is thought to have low toxicity. The FDA (US food and drug administration) has deemed acetone safe in adhesives and food contact coatings. There are no critical health effects from exposure to occasional or intermittent use of products containing acetone. The main primary reason of concern is ingestion of acetone (and acetone-free removers) accidentally in children,” Dr Eisman said.
What does acetone do to nails?
It’s complicated.
Anything you do to your nails other than trimming and buffing them will cause some kind of damage, but both Dr Eisman and Phan agreed the biggest concern with using acetone for nail polish, gel, SNS and acrylic removal is dehydration.
“Nail enamel remover containing acetone can cause nail dryness or brittleness. It can also cause troublesome irritant contact dermatitis (red, dry, itchy, inflamed) of the skin surrounding the nail, which can cause pain and discomfort. Broken skin can also be a portal for infection,” Dr Eisman said.
Phan added, “People often don’t realise the damage we do to our nails is not from the polish, but rather the remover. Acetone exposure can cause your nails, cuticles and the skin around your nails to go red, dry and flaky. Acetone has a huge effect on the cuticle skin, which is a protector of your nail. Cuticle skin will dry out when exposed to acetone (cracking, peeling, bleeding).”
Using acetone to remove gel, SNS and shellac manicures is a whole other ball game.
“Gel, SNS and shellac are strongly adherent to the nail plate so they require soaking the finger tips in acetone (or other solvents) for 10-15 minutes to remove. This prolonged exposure to acetone has been associated with nail splitting (onychoschizia) and white discolouration of the nails (pseudo-leukonychia). It can also be associated with overall thinning and severe brittleness of the nails. After soaking in acetone, the skin of the fingertips is also affected and will immediately look white, due to the fact the skin has dried out,” Dr Eisman said.
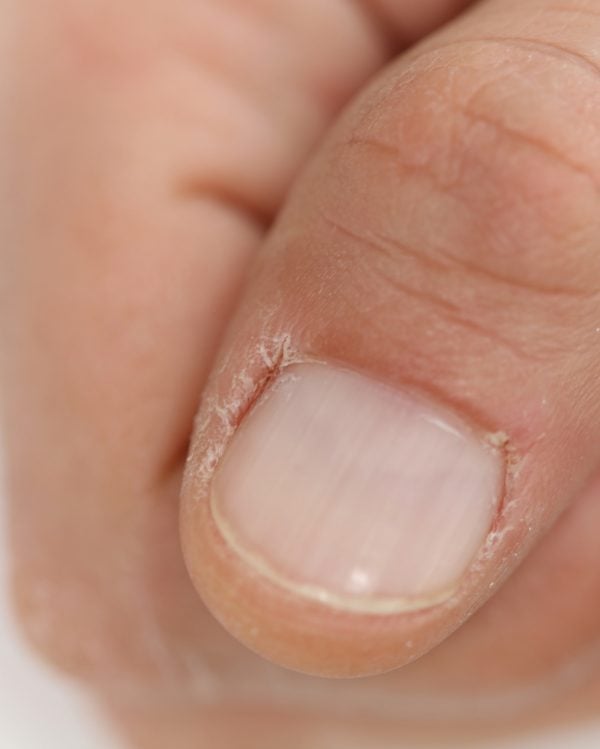 Dry nails and cuticles aren’t fun for anyone. Image: Getty.
Dry nails and cuticles aren’t fun for anyone. Image: Getty.The argument for using acetone to remove nail polish.
Sure, acetone isn’t the purest of solutions going around, but it’s also not the biggest concern in the nail cosmetics industry.
As Dr Eisman explained, there are other nail/manicure practices to be more worried about, like infections from poor salon hygiene, allergic contact dermatitis to acrylates in nail cosmetics and UV lamps.
Acetone is also the most recognised type of nail polish remover and it’s important to note, both acetone and acetone-free removers contain chemicals.
“More recently, acetone-free liquids have been commercialised. This distinction makes some people believe acetone is toxic, but there is no scientific evidence that says acetone is more dangerous than alternative acetone-free solvents. The key ingredient in non-acetone removers is usually a less aggressive and less effective solvent like ethyl acetate made from ethanol and acetic acid,” Dr Eisman said.
“There are many removers on the market that boast being fully ‘natural’ and biodegradable, but even polish removers that say ‘natural’ and ‘organic’ still contain solvents (just not acetone). They usually require more work and effort to remove nail polish and can therefore cause more trauma to the adjacent skin.”
Put simply:
- Acetone nail polish remover = fastest way to remove nail polish and best for glitter/thick/stubborn polishes.
- Non-acetone nail polish remover = more gentle way to remove polish, but will take a bit longer.
- Natural nail polish removers = free from chemicals but will require manual scrubbing to remove polish, which can damage the nail.
So if not acetone, what else?
How to remove nail polish without acetone.
There are a number of acetone-free nail polish removers out there, but as mentioned, they all contain a different chemical solvent.
Phan said The Nail Lab use OPI Expert Touch Lacquer Remover to remove nail polish. This contains 15 per cent acetone, as well as grape seed oil, vitamin E and aloe vera.
How to remove acrylic nails without acetone.
Nail technician Vanessa Schirripa of Pretty Little Things Beauty said the alternative to using acetone to remove acrylic nails is the traditional file and drill technique.
“A nail technician can use nail pliers to help lift the acrylic from the nail bed and use a drill to help scale down the thick acrylic rather than dissolving it. However, this can be a semi-painful way of removing false nails as the drill can give a slight burning sensation on the actual nail. Not for the faint hearted!” she told Mamamia.
How to remove Shellac without acetone.
“Shellac is much thinner than acrylic and can be removed through a peel, drill or buff. Non-acetone products can be used for successful removal, too,” Schirripa said.
“All you need to do is soak a cotton ball in a non-acetone product and wrap it around the shellac polish. Cover with foil and let it soak through for 10 minutes. Then, file or drill off the shellac and you’re done.”
Side note – To help minimise damage, use a nail strengthening product in between every few manicures to keep your nails in good shape.
How to remove SNS nails without acetone.
Schirripa said SNS nails can also be removed with a similar non-acetone soaking process, but as SNS is thicker than shellac, it’ll take a bit longer.
“To remove SNS, soak the nails in an acetone-free remover for 30 minutes or until the gel starts to melt away. Once you feel the nail loosen, gently pull it off with tweezers. Then, you can file, shape and add a clear polish or nail strengthener,” Schirripa said.
A final word on acetone and nail health…
After all that, here are Dr Eisman’s final thoughts:
“Acetone is still the most effective and least traumatic way to remove nail varnish and does not at this stage appear to be toxic or have health risks. Whatever agent you chose, acetone or acetone-free, make sure to moisturise your hands and nails after polish removal.”
Capeesh?
Authored by: Amy Clark
Mamamia Senior Lifestyle Writer
Link to original story: https://www.mamamia.com.au/what-is-acetone-nail-polish-remover/