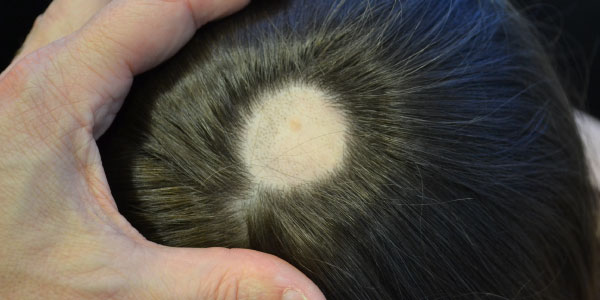
Adults who have a diagnosis of severe alopecia areata (AA) may qualify for this clinical research trial and receive investigational subcutaneous injection or placebo.
- Age: ≥18 years old
- AA diagnosis ≥ 1 year
- 50% or more scalp hair loss
- Current AA episode: ≥ 6 months and ≤ 8 years
- Willing to maintain hair style and hair care such as products, wigs, and extensions. Unable to shave scalp hair 2 weeks before visits.
Patients will be asked to visit the clinic about 12 times over approximately 54 weeks. There is no cost to participate, and participants will be reimbursed for their time and travel.
For further information on the study, please follow the link:
Trial ID: NCT06444451
Accreditations & Memberships





















